N-(3-hydroxy-2-naphthoyl)aniline
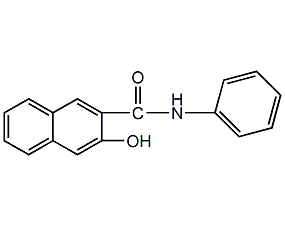
Structural formula
| Business number | 0256 |
|---|---|
| Molecular formula | C17H13NO2 |
| Molecular weight | 263.29 |
| label |
Naphthol AS, Ice dye coupling component 2, Naphthol AS, Ice stain phenol AS, Naphto AS, 3-Hydroxy-N-phenyl-2-naphthalenecarboxamide |
Numbering system
CAS number:92-77-3
MDL number:MFCD00004096
EINECS number:202-188-1
RTECS number:202-188-1
BRN number:1842678
PubChem number:24853271
Physical property data
1. Character: Beige or reddish powder
2. Density (g/mL,25/4℃): Undetermined
3. Relative vapor density (g/mL,AIR=1): Undetermined
4. Melting point (ºC):247 -250
5. Boiling point (ºC,Normal pressure): Undetermined
6. Boiling point (ºC,5.2kPa): Undetermined
7. Refractive Index: Undetermined
8. Flash Point (ºC): Undetermined
9. Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa,25ºC): Undetermined
12. Saturated vapor pressure (kPa,60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (octanol/Log value of the partition coefficient of water: undetermined
17. Explosion upper limit (%,V/V): Undetermined
18. Lower explosion limit (%,V/V): Undetermined
19. Solubility: Insoluble in water and sodium carbonate solution, yellow in sodium hydroxide solution, slightly soluble in ethanol, soluble in hot nitrobenzene.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 80.55
2. Molar volume (m3/mol):200.8
3. Isotonic specific volume (90.2K):565.7
4. Surface Tension (dyne/cm):62.9
5. Polarizability(10-24cm3):31.93
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 9
6. Topological molecule polar surface area 49.3
7. Number of heavy atoms: 20
8. Surface charge: 0
9. Complexity: 338
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
This product should be sealed and stored in a cool place.
Synthesis method
By2,3Acid and aniline are condensed in the presence of phosphorus trichloride and chlorobenzene, and then the chlorobenzene is distilled out. Raw material consumption(kg/t)2,3Acid (100%) 780aniline (99%) 400Phosphorus trichloride (100%) 235Chlorobenzene (98%) 220Soda ash ( 98%) 210
Purpose
Widely used as a primer for dyeing and printing cotton fiber fabrics, such as with yellow base GCCouple is yellow, With orange baseGCCoupling It is orange, with a bright red baseRCor red baseKBCoupled to red, with the big red baseG Coupled to the red color of the national flag, and the blue base VBor blue base BBCoupled to blue, with red baseRCCoupled with sauce red, and maroon red baseGBCcoupled with jujube Red. Can also be used to make fast pigment dyes and organic pigments.
extended-reading:https://www.bdmaee.net/dimethylethanolamine/extended-reading:https://www.morpholine.org/category/morpholine/page/5398/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/75.jpgextended-reading:https://www.cyclohexylamine.net/dabco-tmr-4-trimer-catalyst-tmr-4/extended-reading:https://www.cyclohexylamine.net/pc-cat-td-25-dabco-tertiary-amine-catalyst/extended-reading:https://www.newtopchem.com/archives/45157extended-reading:https://www.bdmaee.net/niax-d-50-tertiary-amine-catalyst-momentive/extended-reading:https://www.bdmaee.net/niax-k-zero-3000-trimer-catalyst-momentive/extended-reading:https://www.cyclohexylamine.net/dabco-dc5le-reaction-type-delayed-catalyst/extended-reading:https://www.bdmaee.net/wp-content/uploads/2016/06/Addocat-108.pdf


