L-arginine
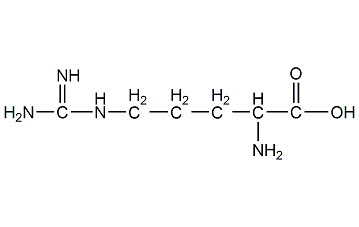
Structural formula
| Business number | 01HJ |
|---|---|
| Molecular formula | C6H14N4O2 |
| Molecular weight | 174.20 |
| label |
2-amino-5-argininoic acid, L-protein amino acids, guanidinepentine, arginine, L-2-amino-arginine acid, L-guanidinine, (S)-2-Amino-5-guanidinopentanoic acid, L-Arginine base, L-2-Amino-5-guanidinopentanoic acid, 2-Amino-5-guanidinopentanoic acid, nutritional supplements, flavor enhancers, intermediates, Biochemical reagents |
Numbering system
CAS number:74-79-3
MDL number:MFCD00002635
EINECS number:200-811-1
RTECS number:CF1934200
BRN number:1725413
PubChem number:24901894
Physical property data
1. Properties: White to slightly yellow crystals or crystalline powder, with special sweet, bitter taste and unique flavor 2. Density (g/mL, 25/4℃): 1.46
3. Relative vapor density (g/mL , air=1): Uncertain
4. Melting point (ºC): 244(dec.)(lit.)
5. Boiling point (ºC, normal pressure): Uncertain
6. Boiling point (ºC, 5.2 kPa): Uncertain
7. Refractive index: 27 ° (C=8, 6mol/L HCl)
8. Flash point (ºC): Uncertain
9. Specific rotation (º): 27.1 º (c=8, 6N HCl)
10. Autoignition point or ignition Temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25 ºC): Uncertain
12. Saturated vapor pressure (kPa, 60 ºC): Uncertain
p>
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain Determine
16. The logarithmic value of the oil-water (octanol/water) partition coefficient: Uncertain
17. The upper limit of explosion (%, V/V): Uncertain
18. Lower explosion limit (%, V/V): Uncertain
19. Solubility: Easily soluble in water (0℃, 83g/L, 20℃, 148.7g/L, 50 ℃, 400g/L), very slightly soluble in ethanol, insoluble in ether
Toxicological data
Mutagenicity: Grasshopper ParentalTEST SYSTEM: 100 mmol/L; human lymphocyteSister chromatid exchange test system: 10 mg/L;
Ecological data
None
Molecular structure data
1. Molar refractive index: 40.69
2. Molar volume (cm3/mol): 118.7
3. Isotonic specific volume (90.2K ): 338.5
4. Surface tension (dyne/cm): 66.1
5. Polarizability (10-24cm3): 16.13
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): -4.2
2. Number of hydrogen bond donors: 4
3. Number of hydrogen bond acceptors: 6
p>
4. Number of rotatable chemical bonds: 5
5. Number of tautomers: 2
6. Topological molecular polar surface area (TPSA): 128
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 176
10. Isotopic atoms Quantity: 0
11. Determine the number of atomic stereocenters: 1
12. Uncertain number of atomic stereocenters: 0
13. Determine the chemical bond establishment Number of stereocenters: 0
14, Number of uncertain chemical bond stereocenters: 0
15, Number of covalent bond units: 1
Properties and stability
1. In the body, it is an intermediate metabolite of ornithine cycle, which can promote the production and excretion of urea, correct ammonia poisoning and relieve hepatic coma. Arginine is also the main component of sperm protein, which can promote sperm production and increase sperm movement energy.
2. Found in tobacco leaves.
3. It is an important component of sperm protein. It crystallizes from water and is dihydrate, which loses water of crystallization at 105ºC. Crystallized stone anhydrate from ethanol.
Storage method
Sealed packaging in brown glass bottles. Store in a dry place protected from light below 4℃.
Synthesis method
1. The industrial production of lysine is mainly based on direct fermentation method, followed by enzymatic method. The direct fermentation method uses mutant strains of microorganisms to ferment starch hydrolyzed sugar, molasses, acetic acid, ethanol, etc. as raw materials to produce L-lysine.
After the fermentation medium is disinfected, the pre-cultured seeds are added, and after culturing at 30°C for about 70 hours, fermentation is carried out, and then the fermentation liquid is heated to 80°C. After 10 minutes, it is cooled and filtered. Use hydrochloric acid to adjust the pH value of the clear liquid to 40, and pass it through the 732 column (first processed into -NH+4 type) at a flow rate of 75 to 80ml/min until the pH value of the effluent reaches 70. Stop adsorption, rinse with distilled water until colorless, then elute with 2mol/L ammonia water, concentrate the eluate to about 12°Bé, then use hydrochloric acid to adjust the pH value to 49, add activated carbon for decolorization, filter while hot, and then Concentrate under reduced pressure, cool and crystallize, and filter. The resulting crystals are lysine hydrochloride. Re-crystallization can obtain refined lysine.
Fermentation of molasses and corn steep liquor ↓Ion exchange of slant strain isolates
Concentration and decolorization→crystallization→recrystallization→drying→product
2.Extraction method

3. Tobacco: BU, 22; Synthesis: It can be obtained by hydrolysis and refining of gelatin. It can also be synthesized chemically.
Purpose
1. The most important use of lysine is as a feed additive, accounting for 95% of annual production. About 3% is used in the food industry as a nutritional supplement to fortify lysine in food. 2% is used in the pharmaceutical industry.
2.Amino acid drugs. It is used for patients with various types of hepatic coma who are contraindicated in taking sodium glutamate and those with viral hepatic alanine aminotransferase abnormalities.
extended-reading:https://www.bdmaee.net/niax-a-337-delayed-tertiary-amine-catalyst-momentive/extended-reading:https://www.newtopchem.com/archives/44424extended-reading:https://www.bdmaee.net/wp-content/uploads/2021/05/2-7.jpgextended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/33.jpgextended-reading:https://www.bdmaee.net/niax-a-99-strongly-foaming-tertiary-amine-catalyst-momentive/extended-reading:https://www.bdmaee.net/22-dimorpholinodiethylether-2/extended-reading:https://www.newtopchem.com/archives/573extended-reading:https://www.bdmaee.net/spraying-composite-amine-catalyst-nt-cat-pt1003-pt1003/extended-reading:https://www.cyclohexylamine.net/catalyst-1027-polyurethane-catalyst-1027/extended-reading:https://www.newtopchem.com/archives/44632


